 |
 |
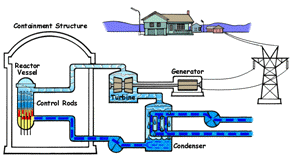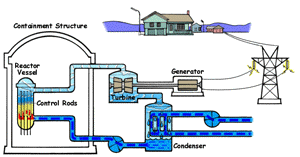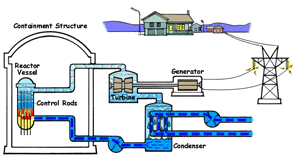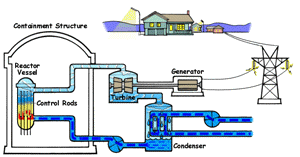
| Boiling Water Reactor (BWR)
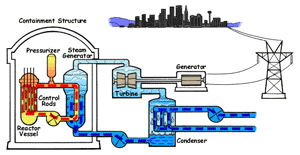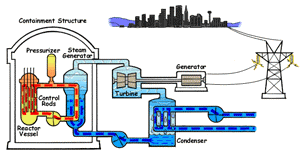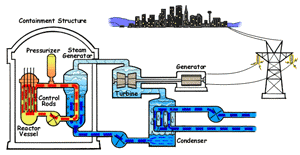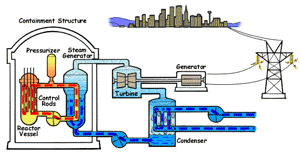
In a boiling water reactor, water is turned into steam by passing through the reactor vessel. This steam turns the turbine which makes the generator produce electricity. The water is then recovered from the steam and passed through to the reactor vessel again. Boiling water reactors are the more dangerous of these two systems, and also produces several times more radioactive waste than a PWR. |
Pressurized Water Reactor (PWR) In a pressurized water reactor, the water which turns the turbine is not passed through the reactor vessel. Instead, a closed-curcuit pressurized water chamber is paired with the reactor, which heats a secondary water cycle. Pressurization ensures that the water heats, but does not boil. This secondary loop is converted to steam and powers the generator, but never reaches the reactor vessel itself. Most reactors in the United States are PWRs. |
There are two common types of reactors in the United States, as illustrated above. In a pressurized water reactor, the steam is condensed to water and is returned to the steam generator. In a boiling water reactor, it returns to the reactor core. There are also gas-cooled reactors which have been in development, including the pebble bed modular reactor (PBMR). The PBMR has a history of failure, and Exelon eventually backed out of supporting the technology. Another common reactor type in North America is the Canadian deterium-uranium model (or CANDU). Pakistani and Indian reactors are all either CANDU reactors or have CANDU components which were provided by the Canadian government. There are several other international designs. Chernobyl was a graphite-moderated reactor, and reactors using graphite have had trouble with graphite fires. Sodium-based cooling systems are used in prototype reactors even though the method is notorious for catching on fire. The terms "light water reactor" and "heavy water reactor" are also employed to differentiate reactors by the type of cooling water used. (the CANDU is a heavy water reactor). US military submarines employ pressurized water reactors, which, unlike civilian reactors, use fuel rods with 20-45% uranium-235 (commercial reactors use closer to 4%). According to some sources, they are even capable of utilizing fuel rods made of up to 90% U-235. Under normal conditions, the reactor's 250 assemblies require refueling every 7-10 years.
A gastight shell or other enclosure around a nuclear reactor makes up a containment structure to assist in confining some fission products that otherwise might be released to the atmosphere in the event of an accident. Many are suprised to hear that the proposed pebble-bed design is planned to not even have a containment structure. There are also breeder reactors, which fission plutonium specifically to generate more plutonium, and have not been used by the DOE for some time. The experimental fast-flux reactors are designed to burn fuel which burns hotter than standard fuel, most specifically mixed oxide fuel (MOx). Despite the fact that working with MOx fuel is extremely dangerous, even compared to normal fuel, some have suggested the possibility of using MOx in aging reactors which were designed for plain uranium oxide.
FissionBasics: Bombs and Plants
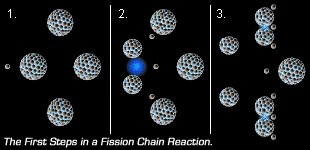 When an uranium-235 atom fissions with some neighboring uranium-235 around, the 3 neutrons released will trigger 3 of it's neighbors to fission. Those 3 triggered fissions will then produce 9 neutrons which will trigger 9 fissions, triggering 27 neutrons, 27 fissions 81 fissions, etc,. This is a nuclear chain reaction, and plutonium fissions almost the same way. In a reactor, control rods absorb neutrons to limit fission reactions. |
Both nuclear power plants and nuclear weapons utilize the same process of atomic fission.
Whereas a nuclear weapon's entire fissionable mass is allowed to chain-react as a whole and explode, a reactor relies on
control rods to moderate how quickly the uranium fissions, slowing the reaction so that an explosion or meltdown does not take
place. So, inside of a nuclear power plant, a contained fission reaction is achieved by using boron or hafnium control rods
to absorb neutrons, manage the chain reaction, and prevent the uranium mass from going supercritical, resulting in a meltdown.
Fuel rods are assembled into bundles called fuel elements or fuel assemblies, which are loaded individually into the reactor
core. Also, in a nuclear explosion, fission products are dispersed by the blast and radioactive fallout. In a nuclear power plant, fission products and neutrons are absorbed by structural metals surrounding the reactor, the control rods, filter sludges, resins, and other materials. All of these materials make up what is refered to as "low-level radioactive waste". The spent fuel rods are considered "high-level" waste. |
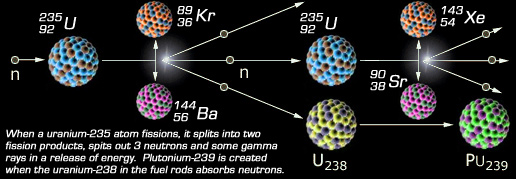
A nuclear power plant is simply a method to produce plutonium, which is the reason reactors were employed in the US to begin with. This is clearly reflected in the US State Department's concern over "third-world" or "axis of evil" countries developing nuclear power plants. Commercial plants in the United States were initially built on slogans such as "too cheap to meter" and "atoms for peace." Since producing electricity with fission has also proved to not be cost effective, these sorts of ideas are stating quite the opposite of the real picture. The proliferation of nuclear power plants had the single purpose to ensure that US stockpiles of plutonium would outnumber that of any other nation, particularly the communist rivals of the US during the Cold War, the USSR and China. Power plants can also maintain fresh supplies of tritium and deuterium, which are needed to build high-megaton hydrogen bombs. Whereas commercial reactors are not offically designated for tritium production, they are employed to the task when the US Government sees the need to do so. In 2002, Tennessee reactors were designated for tritium production starting with the Watts Bar facility. Supposedly, Public Law 97-415 prohibits United States defense use of plutonium produced in commercial facilities, but the DOE maintains several of its own production reactors, and is to care for all US spent fuel in the end.
The Nuclear Fuel Chain
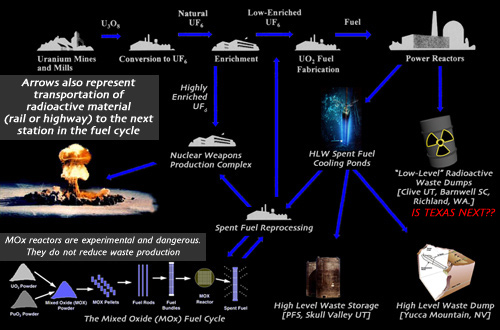
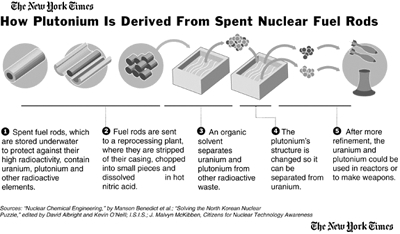
Obtaining fuel for nuclear plants and weapons is lengthy and expensive process. The preliminary procedures from mining the uranium, to enriching the concentration of U-235 and forming usable pellets, are refered to as the "front end" of the fuel chain. There are two uranium enrichment facilities in the US, both gaseous-diffusion plants, one in Paducah, Kentucky, and the Portsmouth plant in Piketon, Ohio. The plants generate massive amounts of air pollution, but also tons of depleted uranium (U-238). After the uranium is enriched in percentages of fissionable U-235, it can either be used in a plant or in a weapon. Aside from spent fuel, everything else nuclear power plants produce is called "low-level" waste, which is sent to 3 of the operating low-level waste dumps in the United States. Spent fuel will either be disposed of or placed in what is refered to as interim storage. An interim storage facility may be later converted to a disposal site, nobody knows for certain. Reprocessing spent fuel yields plutonium and uranium, which can either be used to make Mixed Oxide (MOx) fuel, increase uranium fuel reserves, or make nuclear weapons. Mixed Oxide-ready reactors are still in the development stages, and do not seem to be proceeding as industry and the DOE had hoped.
There is one crucial component of the fuel chain which is located between every station in the diagram, and that is transportation. There is a massive amount of material being moved around throught the entire process.
Nuclear Weapons Overview
There are only a few types of nuclear weapons. The uranium-gun design, which was dropped on Hiroshima, simply slams together two masses of fissionable uranium to start a chain reaction. A hydrogen bomb is a more sophisticated design which employs the fission of plutonium to additionally super-heat either deuterium (hydrogen-2) or tritium (hydrogen-3) to such a degree that a fusion reaction takes place, releasing even more subatomic particles and amplifying the fission chain reaction of the plutonium. This use of hydrogen was refered to as "spiking" or "boosting" the weapon.
In a hydrogen bomb, the plutonium is shaped into a sphere, and the hydrogen mass is placed inside. This sphere is placed between two hemispheres of high-explosives which confine and reflects the initial blast toward the center of the sphere. This chamber is generally refered to as a set of "lenses," although they are not lenses in terms of eyeglasses or telescopes. The term lense is employed for it's meaning of focusing or reflecting the radioactive particles in such a way that the mass implodes and creates a tremendous release of energy. Boosting more than doubles a weapon's yield, and has been a key component of expanding the US stockpile of nuclear weapons.
Grades of Uranium and Plutonium
Natural uranium usually has about 0.7 percent of the fissionable Uranium-235. Low-enriched uranium (LEU) typically contains about 3-7 percent U-235 for reactor operation. Weapons-usable enriched uranium contains more than 20 percent of uranium-235, and the label "weapons-grade" high-enriched uranium (HEU) is usually reserved for uranium made up of over 90 percent of the fissile uranium-235 isotope.
When discussing plutonium, the use of grade assignment is misleading- any amount of plutonium is weapons-usable. There are a number of grades which are stated, such as reactor grade, weapons grade, and special grade. In 1962, the US conducted a successful test which used reactor-grade plutonium in place of weapon-grade plutonium, resulting in a yield of less than 20 kilotons. The test confirmed that reactor-grade plutonium could be used to make a nuclear explosive. The results were declassified in July 1977, however, details such as plutonium quantity and exact yield were sanitized for reasons of national security.
Plutonium-239 is produced when the most common isotope of uranium, uranium-238, absorbs a neutron and then transforms to plutonium. When a reactor is used specifically for creating weapons plutonium, the fuel rods are removed and the plutonium is separated from them after relatively brief irradiation (at low "burnup"). The resulting "weapons-grade" plutonium is typically about 93 percent plutonium-239.
There are some considerations which are made as to the particular isotope to use. For example, plutonium-240 has a high rate of spontaneous fission, meaning that the plutonium in the device will continually produce many background neutrons, which have the potential to reduce weapon yield by starting the chain reaction prematurely. The isotope americium-241 (which results from the 14-year half-life decay of plutonium-241 and builds up in "reactor-grade" plutonium over time) emits highly penetrating gamma rays, increasing the radioactive exposure of any personnel handling the weapon components. Plutonium-238 decays relatively rapidly, thereby significantly increasing the rate of heat generation in the material. Thus, plutonium-239 is the isotope of choice for weapons designers.
All of these grades of plutonium can be used to make nuclear weapons. The only isotopic mix of plutonium which cannot realistically be used for nuclear weapons is nearly pure plutonium-238, which generates so much heat that the weapon would not be stable. (International rules require equal levels of safeguards for all grades of plutonium except plutonium containing more than 80 percent plutonium-238, which need not be safeguarded.
 |
A Natural Reactor Ages Ago in Gabon, Africa
Whereas fission reactions happen constantly in stars like our Sun, it has happened naturally on Earth in at least one extremely rare occassion with very special circumstances. In what is now Gabon in west Africa in 1972, French researchers found a deposit of uranium which had only 0.44% U-235 compared to the normal 0.72%. This indicated that some of the U-235 had undergone spontaneous nuclear fission at some point in the past. Also, fission-produced isotopes of neodynium and samarium were found. Some samples were found with a U-235 concentration as low as 0.29%. Models of the process suggested sustained fission reactions over a period of about a million years during a time period about two billion years ago. The age estimate from cores in the reactor zones suggest a time frame between 1.7 and 1.9 billion years ago. For U-235 (halflife 700 million years) and U-238 (halflife 4.5 billion years), this would give a concentration of about 3% for the U-235 at the time of the reaction. It is presumed that ground water seeping through the ore served as a natural moderator to slow down the fission neutrons.